https://doi.org/10.1140/epjd/s10053-021-00301-6
Regular Article – Clusters and Nanostructures
Adsorption of helium on a charged propeller molecule: hexaphenylbenzene
1
Institut für Ionenphysik und Angewandte Physik, Universität Innsbruck, 6020, Innsbruck, Austria
2
Université Grenoble Alpes, CNRS, LiPhy, 38000, Grenoble, France
3
Laboratory Astrophysics and Cluster Physics Group of the MPI for Astronomy at the University of Jena, Helmholtzweg 3, 07743, Jena, Germany
4
Department of Physics, University of New Hampshire, 03824, Durham, NH, USA
Received:
16
September
2021
Accepted:
4
November
2021
Published online:
29
November
2021
Physisorption on planar or curved graphitic surfaces or aromatic rings has been investigated by various research groups, but in these studies, the substrate was usually strictly rigid. Here, we report a combined experimental and theoretical study of helium adsorption on cationic hexaphenylbenzene (HPB), a propeller-shaped molecule. The orientation of its propeller blades is known to be sensitive to the environment, with substantial differences between the molecule in the gas phase and in the crystalline solid. Mass spectra of He HPB
HPB , synthesized in helium nanodroplets, indicate enhanced stability for ions containing
, synthesized in helium nanodroplets, indicate enhanced stability for ions containing 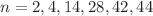 , or 46 helium atoms. Path-integral molecular dynamics simulations reveal a significant dependence of the dissociation energy on the details of the HPB geometry. Good agreement between the experimental data and calculated dissociation energies is obtained, provided that the symmetry of HPB
, or 46 helium atoms. Path-integral molecular dynamics simulations reveal a significant dependence of the dissociation energy on the details of the HPB geometry. Good agreement between the experimental data and calculated dissociation energies is obtained, provided that the symmetry of HPB is reduced from
is reduced from 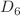 to
to 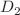 , such a lower symmetry being suggested from quantum chemical calculations as arising upon electron removal.
, such a lower symmetry being suggested from quantum chemical calculations as arising upon electron removal.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1140/epjd/s10053-021-00301-6.
© The Author(s) 2021
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.





