https://doi.org/10.1140/epjd/s10053-021-00142-3
Regular Article - Molecular Physics and Chemical Physics
Confinement effects on C–H and C–F stretching vibrational frequencies of difluoromethane in cold inert gas matrixes: a combined infrared spectroscopy and electronic structure theory study
1
Department of Chemistry, Diamond Harbour Women’s University, Sarisha, West Bengal, India
2
School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, West Bengal, India
Received:
2
December
2020
Accepted:
29
March
2021
Published online:
19
April
2021
Mid-infrared spectra of difluoromethane (DFM) have been recorded by confining the molecule in the matrix cages of pure argon, nitrogen and in the mixed matrixes of argon and nitrogen obtained by mixing the two gases in definite proportions. The measured spectra depict distinct confinement effects in terms of the infrared spectral shifts of the C–H and C–F stretching vibrational fundamentals as compared to the free molecules in the gas-phase, and also some remarkable changes in band appearances and relative intensities. The signs of the spectral shifts are observed to be different for the 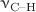 and
and 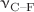 transitions. Pronounced blue shifts are observed for the two
transitions. Pronounced blue shifts are observed for the two 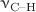 modes in pure nitrogen matrix, which progressively reduce in magnitude in the mixed matrixes, and is least in the pure argon matrix. On the other hand, distinct red shifts of the
modes in pure nitrogen matrix, which progressively reduce in magnitude in the mixed matrixes, and is least in the pure argon matrix. On the other hand, distinct red shifts of the 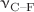 vibrations are evident in pure nitrogen matrix, which are reduced in magnitude in the mixed matrixes, and become even less pronounced in pure argon matrix. The effect of Fermi resonances in the
vibrations are evident in pure nitrogen matrix, which are reduced in magnitude in the mixed matrixes, and become even less pronounced in pure argon matrix. The effect of Fermi resonances in the 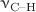 region, that has motivated several previous investigations of DFM including high resolution gas phase studies, becomes explicit upon comparison of the matrix isolation spectrum with the gas phase spectrum. Electronic structure calculations using several DFT and DFT-D methods, in conjunction with NBO analysis, have been carried out for various sizes of
region, that has motivated several previous investigations of DFM including high resolution gas phase studies, becomes explicit upon comparison of the matrix isolation spectrum with the gas phase spectrum. Electronic structure calculations using several DFT and DFT-D methods, in conjunction with NBO analysis, have been carried out for various sizes of 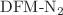 and DFM-Ar complexes in order to understand the underlying interactions responsible for the observed shifts. The calculations are found to satisfactorily reproduce the observed variations in magnitude and direction of the shifts of both the
and DFM-Ar complexes in order to understand the underlying interactions responsible for the observed shifts. The calculations are found to satisfactorily reproduce the observed variations in magnitude and direction of the shifts of both the 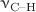 and
and 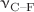 transitions for the change of the medium. It is inferred that the inert gas matrix environment exerts an electronic re-organization within the DFM molecule, and the consequent rehybridization of the carbon-centric hybrid orbital of the C–H and C–F bonds is responsible for the observed spectral shifts.
transitions for the change of the medium. It is inferred that the inert gas matrix environment exerts an electronic re-organization within the DFM molecule, and the consequent rehybridization of the carbon-centric hybrid orbital of the C–H and C–F bonds is responsible for the observed spectral shifts.
Atoms and Molecules in a Confined Environment—edited by C. N. Ramachandran, Vincenzo Aquilanti, Henry Ed Montgomery, N. Sathyamurthy.
© The Author(s), under exclusive licence to EDP Sciences, SIF and Springer-Verlag GmbH Germany, part of Springer Nature 2021





