https://doi.org/10.1140/epjd/s10053-025-01023-9
Regular Article - Atomic and Molecular Collisions
Electron interaction with laser-desorbed thymidine and guanine in the gas phase
1
Institut für Ionenphysik und Angewandte Physik, Universität Innsbruck, Technikerstrasse 25, 6020, Innsbruck, Austria
2
Center for Molecular Biosciences Innsbruck, Universität Innsbruck, Technikerstrasse 25, 6020, Innsbruck, Austria
Received:
22
April
2025
Accepted:
5
June
2025
Published online:
30
June
2025
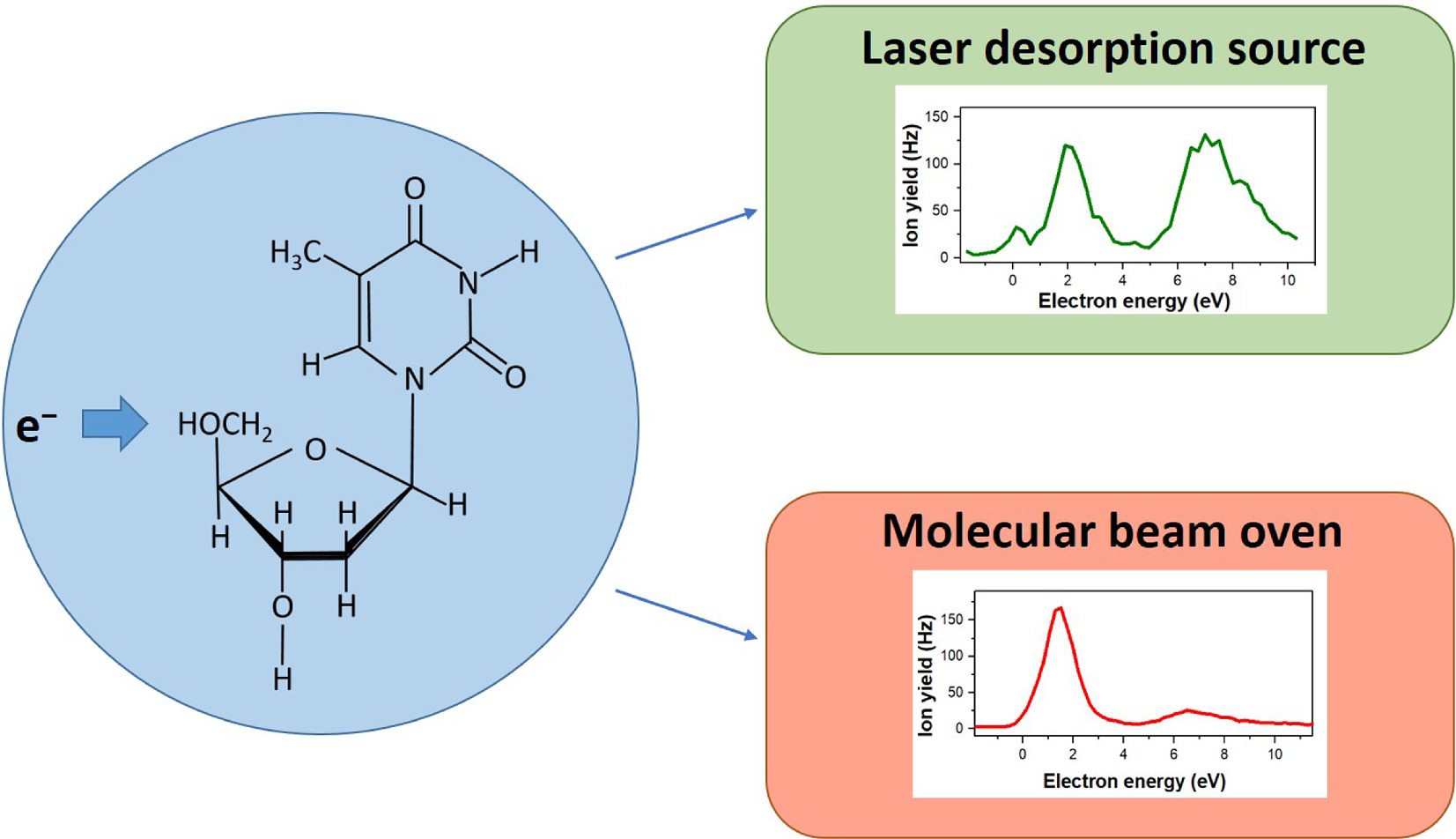
In the present study we investigated electron attachment to the nucleoside thymidine (Td) and the nucleobase guanine (G) using a laser desorption source to transfer the compounds into the gas phase. Previous studies with Td indicated that the compound is thermally labile and may degrade upon thermal heating in standard molecular beam source. The present negative ion mass spectra for laser-desorbed Td and resistively heated Td share the same most three abundant fragment anions. Among those is the dehydrogenated parent anion (Td-H)− which is strongly enhanced for laser-desorbed Td. We also find a considerable change of the fragmentation pattern for less abundant peaks in the mass spectra as well as changed characteristic in the total and mass selected anion efficiency curves of fragment anions. Electron attachment to G proceeds predominantly at electron energies below 3 eV. We ascribe this property to formation of a dipole-bound anion acting as a precursor state for efficient formation of the dehydrogenated anion (G-H)−. The present results complement previous electron attachment studies with other nucleobases showing that the dehydrogenated parent anion is the most abundant fragment anion for G as well.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1140/epjd/s10053-025-01023-9.
© The Author(s) 2025
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.