https://doi.org/10.1140/epjd/s10053-024-00899-3
Regular Article - Atomic and Molecular Collisions
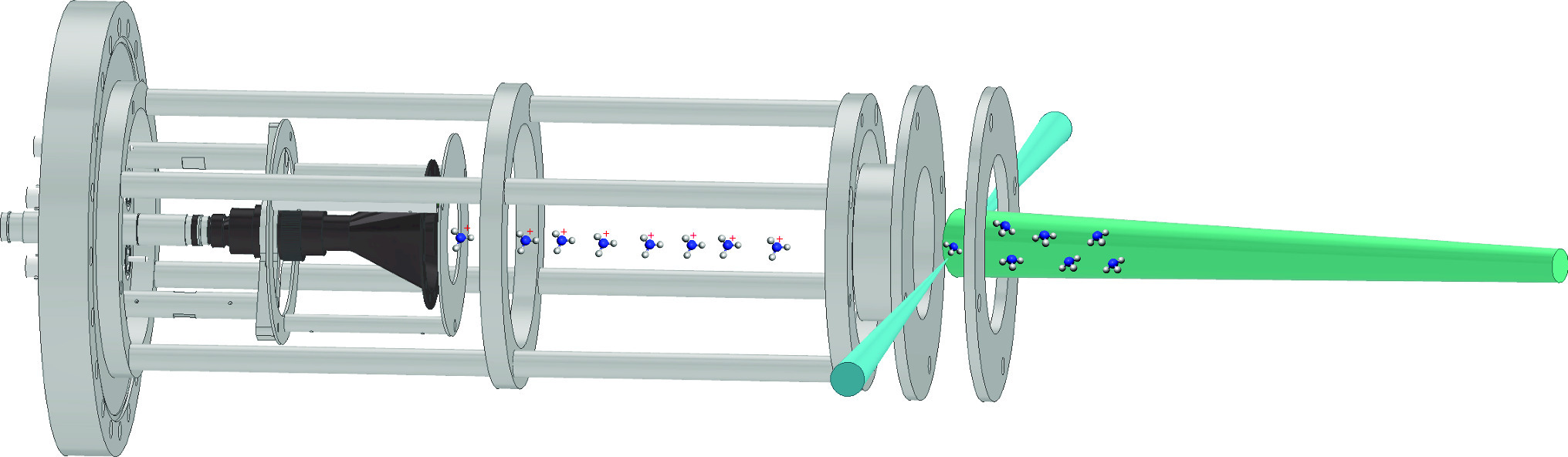
The influence of experimental conditions on absolute beam density measurements for NH and H
and H
1
Department of Physics, University of Liverpool, L69 7ZE, Liverpool, UK
2
Physical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, OX1 3QZ, Oxford, UK
a
rahul.pandey@liverpool.ac.uk
e
b.r.heazlewood@liverpool.ac.uk
Received:
23
May
2024
Accepted:
23
July
2024
Published online:
20
August
2024
In order to establish important reaction properties, such as rate coefficients, it is often necessary to know the number of reactants that are present in an interaction region. The absolute number densities of pulsed supersonic molecular (NH ) and atomic (H) beams are reported using a laser-based detection method, under a range of experimental conditions including photolysis, Zeeman deceleration, and magnetic focusing. Time-averaged densities of (3.6 ± 2.7)
) and atomic (H) beams are reported using a laser-based detection method, under a range of experimental conditions including photolysis, Zeeman deceleration, and magnetic focusing. Time-averaged densities of (3.6 ± 2.7)  10
10 cm
cm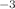 are reported for successfully Zeeman-decelerated and magnetically focused H atoms, generated by the photodissociation of precursor NH
are reported for successfully Zeeman-decelerated and magnetically focused H atoms, generated by the photodissociation of precursor NH molecules. Without the magnetic guide components in the beamline, the density of the target radicals of interest is somewhat lower, at (2.5 ± 1.8)
molecules. Without the magnetic guide components in the beamline, the density of the target radicals of interest is somewhat lower, at (2.5 ± 1.8)  10
10 cm
cm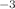 . The average density of the undecelerated H atom beam is approximately an order of magnitude higher (2.9 ± 1.9)
. The average density of the undecelerated H atom beam is approximately an order of magnitude higher (2.9 ± 1.9)  10
10 cm
cm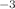 , with the average density of the molecular ammonia beam over two orders of magnitude higher again (5.1 ± 2.9)
, with the average density of the molecular ammonia beam over two orders of magnitude higher again (5.1 ± 2.9)  10
10 cm
cm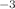 . The average number densities measured for the two different species of interest in this work span more than three orders of magnitude. These findings highlight the need for accurate and precise experimental measurements of number densities—for each species of interest, under the appropriate experimental conditions—before doing absolute rate coefficient calculations.
. The average number densities measured for the two different species of interest in this work span more than three orders of magnitude. These findings highlight the need for accurate and precise experimental measurements of number densities—for each species of interest, under the appropriate experimental conditions—before doing absolute rate coefficient calculations.
Rahul Pandey and Lok Yiu Wu have contributed equally to this work.
© The Author(s) 2024
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.