https://doi.org/10.1140/epjd/s10053-021-00176-7
Regular Article - Atomic and Molecular Collisions
Confinement of  inside carbon nanotubes
inside carbon nanotubes
1
Dipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, Perugia, Italy
2
Department of Chemistry, Indonesia Defense University, Kampus Unhan Komplek IPSC Sentul, Bogor, Indonesia
3
Department of Chemistry, IPB University, Kampus IPB Dramaga, Bogor, Indonesia
Received:
16
December
2020
Accepted:
11
May
2021
Published online:
26
May
2021
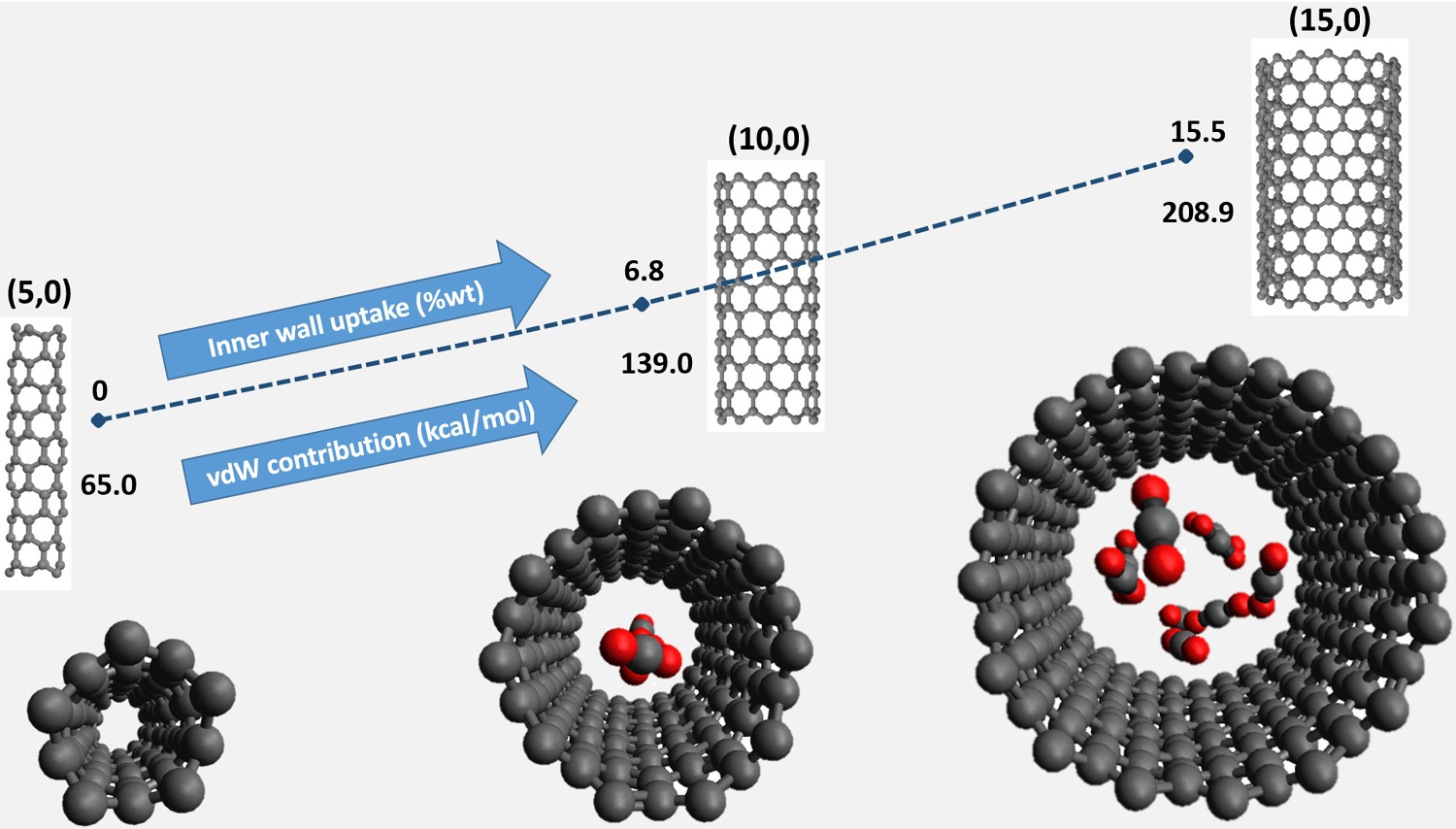
We propose a preliminary study based on molecular dynamics calculations to investigate the adsorption of pure  on flexible single-walled carbon nanotubes (SWCNTs) of different sizes. The adsorption capacities of SWCNTs were simulated and the effect of chirality and diameter of SWCNTs was assessed, to check them as sizable carbon structured materials suitable for
on flexible single-walled carbon nanotubes (SWCNTs) of different sizes. The adsorption capacities of SWCNTs were simulated and the effect of chirality and diameter of SWCNTs was assessed, to check them as sizable carbon structured materials suitable for  confinement and storage. The potential energy surfaces, describing the intermolecular interactions involving
confinement and storage. The potential energy surfaces, describing the intermolecular interactions involving  -SWCNT and
-SWCNT and  -
- pairs, have been described specifically by adopting the improved lennard jones model potential. The intramolecular interactions within the SWCNT were considered explicitly since they are responsible for out-of-plane movements of carbon atoms and the flexibility of nanotubes. These well-formulated potentials are well capable of defining
pairs, have been described specifically by adopting the improved lennard jones model potential. The intramolecular interactions within the SWCNT were considered explicitly since they are responsible for out-of-plane movements of carbon atoms and the flexibility of nanotubes. These well-formulated potentials are well capable of defining  confinement through physisorption and guarantee a quantitative description and realistic results for the dynamics of the interactions. The flexible SWCNTs can adsorb up to 35 wt% at 273 K, a property that makes them potentially versatile materials competitive with other carbon-derived adsorbents to cope with
confinement through physisorption and guarantee a quantitative description and realistic results for the dynamics of the interactions. The flexible SWCNTs can adsorb up to 35 wt% at 273 K, a property that makes them potentially versatile materials competitive with other carbon-derived adsorbents to cope with  gas emission.
gas emission.
© The Author(s) 2021
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.