https://doi.org/10.1140/epjd/e2002-00168-1
Barrier-free intermolecular proton transfer in the uracil-glycine complex induced by excess electron attachment
1
Environmental Molecular Sciences Laboratory, Theory, Modeling & Simulation, Pacific
Northwest National Laboratory, Richland, WA 99352, USA
2
Department of Chemistry, University of Gdańsk, Sobieskiego 18, 80-952 Gdańsk, Poland
3
Department of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA
Corresponding authors: a maciej.gutowski@pnl.gov - b kitbowen@jhu.edu
Received:
6
April
2002
Published online:
13
September
2002
The photoelectron spectra (PES) of anions of uracil-glycine and uracil-phenylalanine complexes
reveal broad features with maxima at 1.8 and 2.0 eV. The results of ab initio density functional
B3LYP and second order Møller-Plesset theory calculations indicate that the excess electron
occupies a 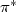 orbital localized on uracil. The excess electron attachment to the complex can
induce a barrier-free proton transfer (BFPT) from the carboxylic group of glycine to the O8 atom
of uracil. As a result, the four most stable structures of the anion of uracil-glycine complex can be characterized as the neutral radical of hydrogenated uracil solvated by the anion of deprotonated glycine. The similarity between the PES spectra for the uracil complexes with glycine and
phenylalanine suggests that the BFPT is also operative in the case of the latter anionic species.
The BFPT to the O8 atom of uracil may be related to the damage of nucleic acid bases by low
energy electrons because the O8 atom is involved in a hydrogen bond with adenine in the
standard Watson-Crick pairing scheme.
orbital localized on uracil. The excess electron attachment to the complex can
induce a barrier-free proton transfer (BFPT) from the carboxylic group of glycine to the O8 atom
of uracil. As a result, the four most stable structures of the anion of uracil-glycine complex can be characterized as the neutral radical of hydrogenated uracil solvated by the anion of deprotonated glycine. The similarity between the PES spectra for the uracil complexes with glycine and
phenylalanine suggests that the BFPT is also operative in the case of the latter anionic species.
The BFPT to the O8 atom of uracil may be related to the damage of nucleic acid bases by low
energy electrons because the O8 atom is involved in a hydrogen bond with adenine in the
standard Watson-Crick pairing scheme.
PACS: 31.10.+z – Theory of electronic structure, electronic transitions, and chemical binding / 33.80.Eh – Autoionization, photoionization, and photodetachment / 36.40.Wa – Charged clusters
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag, 2002




