https://doi.org/10.1140/epjd/s10053-024-00810-0
Regular Article - Atomic Physics
Many-body theory calculations of positron binding to hydrogen cyanide
School of Mathematics and Physics, Queen’s University Belfast, University Road, BT7 1NN, Belfast, Northern Ireland, UK
a
jhofierka01@qub.ac.uk
c
d.green@qub.ac.uk
Received:
25
October
2023
Accepted:
28
January
2024
Published online:
3
April
2024
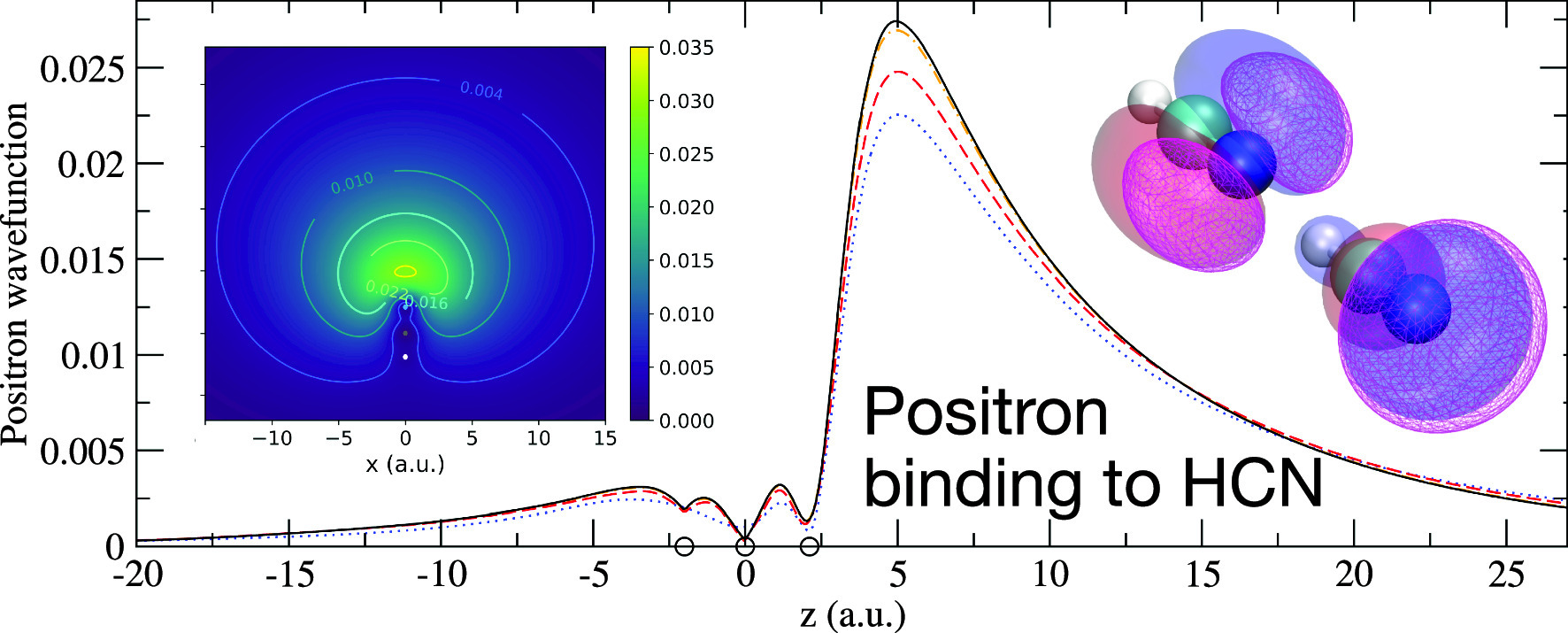
Positron bound state properties in hydrogen cyanide are studied via many-body theory calculations that account for strong positron-electron correlations including positron-induced polarization, screening of the electron–positron Coulomb interaction, virtual-positronium formation and positron–hole repulsion. Specifically, the Dyson equation is solved using a Gaussian basis, with the positron self-energy in the field of the molecule calculated using the Bethe–Salpeter equations for the two-particle and particle–hole propagators. The present results suggest near cancellation of screening corrections to the bare polarization, and the non-negligible role of the positron–hole interaction. There are no existing measurements to compare to for HCN. Previous configuration interaction (CI) and fixed-node diffusion Monte Carlo (FN-DMC) calculations give positron binding energies in the range 35–44 meV, most of which used a single even-tempered basis centred near the nitrogen atom. Using a similar single-centre positron basis we calculate a positron binding energy of 41 meV, in good agreement. However, we find that including additional basis centres gives an improved description of the positron wave function near the nuclei, and results in a converged binding energy in the range 63–73 meV (depending on geometry and approximation to the positron–molecule correlation potential used).
© The Author(s) 2024
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.