https://doi.org/10.1140/epjd/s10053-023-00687-5
Regular Article
Electron scattering on molecular nitrogen: common gas, uncommon cross sections
1
Institute of Plasma Technology, Korea Institute of Fusion Energy (KFE), 37, Dongjangsan-ro, 54044, Gunsan, Jeollabuk-do, South Korea
2
Department of Physics, Chungnam National University, 34134, Daejeon, South Korea
3
Faculty of Physics, Astronomy and Applied Informatics, Institute of Physics, Nicolaus Copernicus University, 87100, Toruń, Poland
4
Department of Physics, University of Central Florida, 32816, Orlando, FL, USA
5
Department of Physics and Astronomy, University College London, WC1E 6BT, London, UK
Received:
1
April
2023
Accepted:
26
May
2023
Published online:
14
June
2023
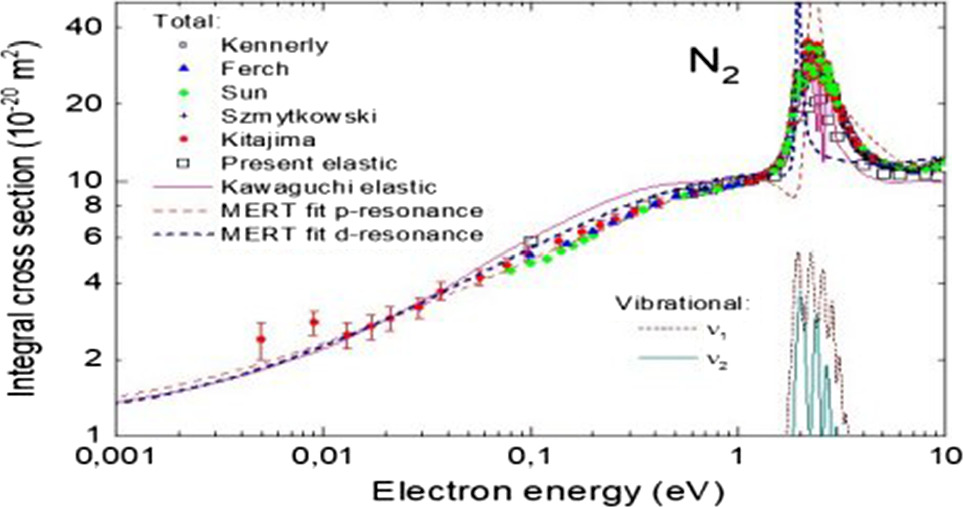
We discuss peculiar features of electron scattering on the N2 molecule and the N2+ ion, that are important for modeling plasmas, Earth’s and other planets’ atmospheres. These features are, among others: the resonant enhancement of the vibrational excitation in the region of the shape resonance around 2.4 eV, the resonant character of some of electronic excitation channels (and high values of these cross sections, both for triplet and singlet states), high cross section for the dissociation into neutrals, high cross sections for elastic scattering (and electronic transitions) on metastable states. For the N2+ ion we discuss both dissociation and the dissociative ionization, leading to the formation of atoms in excited states, and dissociative recombination which depends strongly on the initial vibrational state of the ion. We conclude that the theory became an indispensable completion of experiments, predicting many of partial cross sections and their physical features. We hope that the data presented will serve to improve models of nitrogen plasmas and atmospheres.
© The Author(s) 2023
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.