https://doi.org/10.1140/epjd/s10053-021-00310-5
Regular Article – Atomic and Molecular Collisions
Molecular synthesis in ices triggered by dissociative electron attachment to carbon monoxide
Fachbereich 2 (Chemistry/Biology), Institute for Applied and Physical Chemistry, University of Bremen, Leobener Straße 5, 28359, Bremen, Germany
Received:
1
September
2021
Accepted:
10
November
2021
Published online:
2
December
2021
Chemical reactions in mixed molecular ices as relevant in the context of astrochemistry can be initiated by electron-molecule interactions. Dissociative electron attachment (DEA) as initiating step is identified from the enhancement of product yields upon irradiation at particular electron energies. Herein, we show that DEA to CO leads to the formation of HCN in mixed CO/ ice at electron energies around 11 eV and 16 eV. We propose that this reaction proceeds via insertion of the neutral C fragment into a N–H bond. In the case of CO/
ice at electron energies around 11 eV and 16 eV. We propose that this reaction proceeds via insertion of the neutral C fragment into a N–H bond. In the case of CO/ O and CO/
O and CO/ OH ices, a resonant enhancement of the yields of HCOOH and
OH ices, a resonant enhancement of the yields of HCOOH and  OCHO, respectively, is observed around 10 eV. In both ices, both molecular constituents exhibit DEA processes in this energy range so that the energy-dependent product yield alone does not uniquely identify the relevant DEA channel. However, we demonstrate by comparing with earlier results on mixed ices where CO is replaced by
OCHO, respectively, is observed around 10 eV. In both ices, both molecular constituents exhibit DEA processes in this energy range so that the energy-dependent product yield alone does not uniquely identify the relevant DEA channel. However, we demonstrate by comparing with earlier results on mixed ices where CO is replaced by 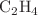 that DEA to CO is again responsible for the enhanced product formation. In this case,
that DEA to CO is again responsible for the enhanced product formation. In this case, 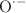 activates
activates  O or
O or  OH which leads to the formation of larger products. We thus show that DEA to CO plays an important role in electron-induced syntheses in molecular ices.
OH which leads to the formation of larger products. We thus show that DEA to CO plays an important role in electron-induced syntheses in molecular ices.
The original online version of this article was revised due to one wrong formula and incorrect in-text citations.
A correction to this article is available online at https://doi.org/10.1140/epjd/s10053-021-00327-w.
Copyright comment corrected publication 2021
© The Author(s) 2021. corrected publication 2021
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.





