https://doi.org/10.1140/epjd/e2014-40423-2
Regular Article
Investigation of aniline by high pressure Raman scattering spectroscopy and quantum chemical calculation
1
College of Physics, Jilin University, Changchun
130012, P.R.
China
2
State Key Laboratory of Superhard Materials, Institute of Atomic
and Molecular Physics, Jilin University, Changchun
130012, P.R.
China
a
e-mail: chenglin@jlu.edu.cn
b
e-mail: mzhou@jlu.edu.cn
Received: 17 July 2013
Received in final form: 23 October 2013
Published online: 27 March 2014
The unexpected redshifts of the NH and the blueshifts of the CH stretching frequencies with increasing pressure were observed in the pressure dependent Raman spectra of aniline at 0−7.7 GPa. Based on the natural bond orbital (NBO) analysis, the opposite frequency shifts of the CH and NH vibrations are attributed to the different pressure-induced change in the electronic antibonding occupancies of NH and CH. Further, the antibonding occupancy of
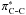 and the charge of phenyl ring in aniline were evaluated, characterizing an increase of antibonding occupancy of
and the charge of phenyl ring in aniline were evaluated, characterizing an increase of antibonding occupancy of
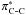 and a decrease of phenyl ring’s charge with increasing pressure. These results reflect that the NH-π and CH-π bonds, as the research target of intermolecular interaction, are becoming weaker as the pressure increases. The research would improve the understanding of the pressure effect on the intermolecular interactions of aniline.
and a decrease of phenyl ring’s charge with increasing pressure. These results reflect that the NH-π and CH-π bonds, as the research target of intermolecular interaction, are becoming weaker as the pressure increases. The research would improve the understanding of the pressure effect on the intermolecular interactions of aniline.
Key words: Molecular Physics and Chemical Physics
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag 2014




