https://doi.org/10.1140/epjd/e2003-00194-5
Novel metal-encapsulated caged clusters of silicon and germanium
Institute for Materials Research, Tohoku University,
2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan and
Dr. Vijay Kumar Foundation, 45 Bazaar Street,
K.K. Nagar (West), Chennai 600 078, India
Corresponding author: a kumar@imr.edu
Received:
10
September
2002
Published online:
3
July
2003
We report the recent findings of metal (M) encapsulated clusters of silicon from
computer experiments based on ab initio total energy calculations and a cage
shrinkage and atom removal approach. Our results show that using a guest atom, it is
possible to wrap silicon in fullerenelike (f) structures, as sp2 bonding is not
favorable to produce empty cages unlike for carbon. Transition M atoms have a
strong bonding with the silicon cage that are responsible for the compact structures.
The size and structure of the cage change from 14 to 20 Si atoms depending upon the size
and valence of the M atom. Fewer Si atoms lead to relatively open structures. We find
cubic, f, Frank-Kasper (FK) polyheral type, decahedral, icosahedral and hexagonal
structures for M@Sin with 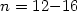 and several different M atoms. The magic
behavior of 15 and 16 atom Si cages is in agreement with experiments. The FK polyhedral
cluster, M@Si16 has an exceptionally large density functional gap of about 2.35 eV
calculated within the
generalized gradient approximation. It is likely to give rise to visible
luminescence in these clusters. The cluster-cluster interaction is weak that makes such
clusters attractive for cluster assembled materials. Further studies to stabilize
Si20 cage with M = Zr, Ba, Sr, and Pb show that in all
cases there is a distortion of the f cage. Similar studies on M encapsulated
germanium clusters show FK polyhedral and decahedral isomers to be more favorable.
Also perfect icosahedral M@Ge12 and M@Sn12 clusters have been obtained with
large gaps by doping with divalent M atoms. Recent results of the H interaction with
these clusters, hydrogenated silicon fullerenes as well as assemblies of clusters
such as nanowires and nanotubes are briefly presented.
and several different M atoms. The magic
behavior of 15 and 16 atom Si cages is in agreement with experiments. The FK polyhedral
cluster, M@Si16 has an exceptionally large density functional gap of about 2.35 eV
calculated within the
generalized gradient approximation. It is likely to give rise to visible
luminescence in these clusters. The cluster-cluster interaction is weak that makes such
clusters attractive for cluster assembled materials. Further studies to stabilize
Si20 cage with M = Zr, Ba, Sr, and Pb show that in all
cases there is a distortion of the f cage. Similar studies on M encapsulated
germanium clusters show FK polyhedral and decahedral isomers to be more favorable.
Also perfect icosahedral M@Ge12 and M@Sn12 clusters have been obtained with
large gaps by doping with divalent M atoms. Recent results of the H interaction with
these clusters, hydrogenated silicon fullerenes as well as assemblies of clusters
such as nanowires and nanotubes are briefly presented.
PACS: 73.22.-f – Electronic structure of nanoscale materials: clusters, nanoparticles, nanotubes, and nanocrystals / 71.15.Nc – Total energy and cohesive energy calculations
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag, 2003




