https://doi.org/10.1140/epjd/e2002-00146-7
Infrared ion dip and ultraviolet spectroscopy of 4-phenyl imidazole, its tautomer, 5-phenyl imidazole, and its multiply hydrated clusters
Physical and Theoretical Chemistry Laboratory, South Parks Road,
Oxford OX1 3QZ, UK
Corresponding author: a john.simons@chem.ox.ac.uk
Received:
20
December
2001
Published online:
13
September
2002
Mass-resolved resonant two photon ionisation (R2PI) and infrared ion dip spectra
have been recorded for 4-phenylimidazole (4PI) and its singly and multiply hydrated
clusters 4PI(H2O)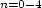 , under supersonic expansion conditions. In the case of
4PI(H2O)
, under supersonic expansion conditions. In the case of
4PI(H2O)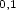 , it has also been possible to record infrared spectra in both the ground
(S0) and excited (S1) states. Combining the experimental data with the results of ab initio calculations has led to the structural assignment of each cluster. In each case,
the water molecules bind primarily to the NH site of the imidazole ring. Clusters with
, it has also been possible to record infrared spectra in both the ground
(S0) and excited (S1) states. Combining the experimental data with the results of ab initio calculations has led to the structural assignment of each cluster. In each case,
the water molecules bind primarily to the NH site of the imidazole ring. Clusters with
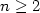 incorporate linear water chains, in which the proton donating terminus bridges
either to the π-electron system (n=2) or to the >N: atom site (n=3,4) on the imidazole
ring. Despite the creation of a “water wire”, connecting the donor and acceptor sites
of imidazole, there is no evidence of proton transfer in either the ground or excited
state.
incorporate linear water chains, in which the proton donating terminus bridges
either to the π-electron system (n=2) or to the >N: atom site (n=3,4) on the imidazole
ring. Despite the creation of a “water wire”, connecting the donor and acceptor sites
of imidazole, there is no evidence of proton transfer in either the ground or excited
state.
PACS: 36.40.Mr – Spectroscopy and geometrical structure of clusters
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag, 2002




