https://doi.org/10.1140/epjd/e2002-00147-6
Interaction between aromatic amine cations and nonpolar solvents: Infrared spectra of isomeric aniline+–Arn (n = 1,2) complexes
Institut für Physikalische Chemie, Universität Basel,
Klingelbergstrasse 80, 4056 Basel, Switzerland
Corresponding author: a otto.dopfer@unibas.ch
Received:
4
February
2002
Published online:
13
September
2002
Infrared (IR) photodissociation spectra of the aniline+–Arn cations, An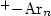 (n=1,2), are
analyzed in the vicinity of the N–H stretch fundamentals. The complexes are produced in an
electron impact (EI) ion source which produces predominantly the most stable cluster
isomers. Two isomers of are identified by their characteristic N–H stretch frequencies:
the planar proton-bound global minimum, in which the Ar ligand forms a nearly linear H-bond
to the amino group, and the less stable local minimum, in which the Ar atom is
attached to the π-electron system of the aromatic ring. This result is the first unambiguous
detection of the most stable dimer. All previous spectroscopic studies of
An+–Ar employed resonance enhanced multiphoton ionization (REMPI) of neutral and
identified only the less stable cation due to restrictions arising from the
Franck-Condon principle. The EI-IR spectrum of shows that the most stable structure
of this trimer features two equivalent H-bonds (
(n=1,2), are
analyzed in the vicinity of the N–H stretch fundamentals. The complexes are produced in an
electron impact (EI) ion source which produces predominantly the most stable cluster
isomers. Two isomers of are identified by their characteristic N–H stretch frequencies:
the planar proton-bound global minimum, in which the Ar ligand forms a nearly linear H-bond
to the amino group, and the less stable local minimum, in which the Ar atom is
attached to the π-electron system of the aromatic ring. This result is the first unambiguous
detection of the most stable dimer. All previous spectroscopic studies of
An+–Ar employed resonance enhanced multiphoton ionization (REMPI) of neutral and
identified only the less stable cation due to restrictions arising from the
Franck-Condon principle. The EI-IR spectrum of shows that the most stable structure
of this trimer features two equivalent H-bonds (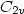 symmetry). The interpretation of the
experimental data is supported by quantum chemical calculations. The ab initio potential of
An+–Ar calculated at the UMP2/6-311G(2df, 2pd) level features global minima
(
symmetry). The interpretation of the
experimental data is supported by quantum chemical calculations. The ab initio potential of
An+–Ar calculated at the UMP2/6-311G(2df, 2pd) level features global minima
(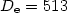 cm-1) and local minima (
cm-1) and local minima (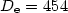 cm-1), with a barrier of
cm-1), with a barrier of 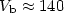 cm-1 for
isomerization from the toward the minimum.
cm-1 for
isomerization from the toward the minimum.
PACS: 36.40.Mr – Spectroscopy and geometrical structures of clusters / 36.40.Wa – Charged clusters / 34.20.Gj – Intermolecular and atom-molecule potentials and forces
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag, 2002





