https://doi.org/10.1007/s100530050530
Ab initio molecular dynamics study on Agn (n = 4, 5, 6)
1
Department of Chemistry, The Chinese University of Hong Kong, Shatin, Hong Kong, P.R. China
2
Steacie Institute for Molecular Sciences, National Research Council of Canada, Ottawa, Ontario K1A 0R6,
Canada
3
Institut für Theoretische Physik, Technische Universität Wien, Vienna, Austria
Received:
5
May
1999
Revised:
20
August
1999
Published online: 15 April 2000
Ab initio Molecular Dynamics (MD) method, based on density
functional theory (DFT) with planewaves and
pse d9126.udopotentials, was used to study the stability and
internal motion in silver cluster Agn, with 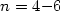 .
Calculations on the
neutral, cationic and anionic silver dimer Ag2 show
that the bond distance and vibrational frequency calculated by DFT
are of good quality. Simulations of Ag4, Ag5, and
Ag6 in canonical ensemble reveal distinct
characteristics and isomerization paths for each cluster.
At a temperature of 800 K, an Ag4 has no definite structure
due to internal motion, while for Ag5 and Ag6 the clusters
maintain the planar structure, with atomic rearrangement
observed for Ag5 but not for Ag6. At
a temperature of 200 K, Ag4 can exist in two planar
structures whilst Ag5 is found to be stable only in the planar
form. In contrast Ag6 is stable in both planar trigonal and 3D
pentagonal structures. Micro-canonical MD
simulation was performed for all three clusters to obtain the
vibrational density of states (DOS).
.
Calculations on the
neutral, cationic and anionic silver dimer Ag2 show
that the bond distance and vibrational frequency calculated by DFT
are of good quality. Simulations of Ag4, Ag5, and
Ag6 in canonical ensemble reveal distinct
characteristics and isomerization paths for each cluster.
At a temperature of 800 K, an Ag4 has no definite structure
due to internal motion, while for Ag5 and Ag6 the clusters
maintain the planar structure, with atomic rearrangement
observed for Ag5 but not for Ag6. At
a temperature of 200 K, Ag4 can exist in two planar
structures whilst Ag5 is found to be stable only in the planar
form. In contrast Ag6 is stable in both planar trigonal and 3D
pentagonal structures. Micro-canonical MD
simulation was performed for all three clusters to obtain the
vibrational density of states (DOS).
PACS: 36.40.-c – Atomic and molecular clusters / 31.15.Qg – Molecular dynamics and other numerical methods / 31.15.Ar – Ab initio calculations
© EDP Sciences, Società Italiana di Fisica, Springer-Verlag, 2000





